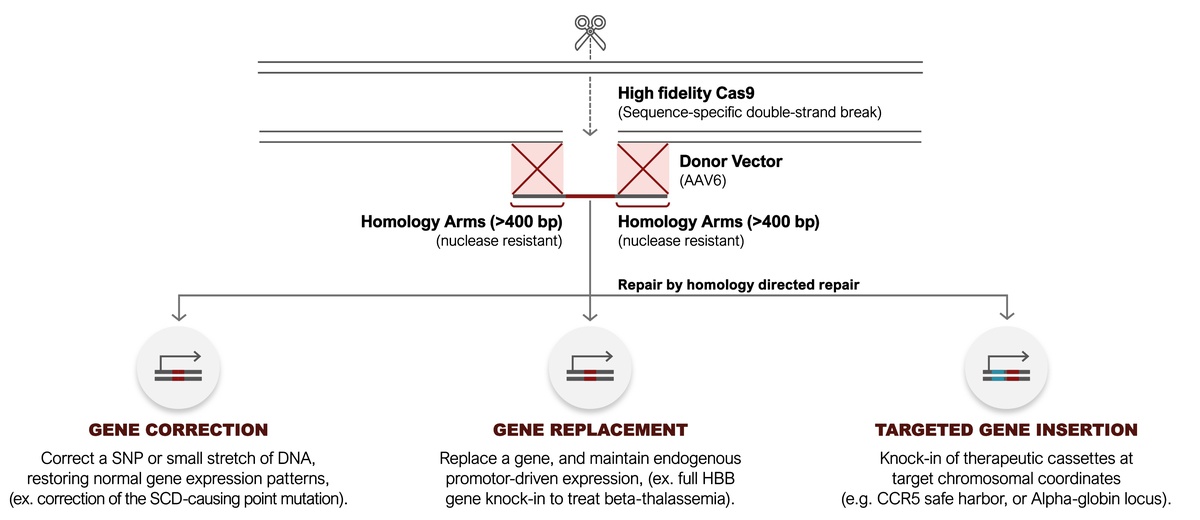
Our technology builds on first-generation proven CRISPR technology to achieve high rates of targeted gene integration. Our next-generation gene editing platform allows us to precisely correct mutations, replace entire disease-causing genes with normal genes, or insert new genes into predetermined, safe locations. Our platform builds on first generation CRISPR-Cas9 technology by not only cutting DNA but providing a template to repair DNA. Our platform HDR-editing technology drives the field of genome editing towards precision genome editing, with the ability to edit kilobase length sequences within genes.

Our approach operates similarly to a “find & replace” system. We utilize CRISPR to locate a target gene and Homology Directed Repair (HDR) to substitute DNA in the target gene with DNA replicated from a template. The HDR process involves employing a high-fidelity ribonucleoprotein (RNP) complex, introduced into a hematopoietic stem cell (HSC). The RNP complex precisely cuts the DNA in the desired area. Subsequently, an AAV6 carrying new, corrected DNA is introduced, delivering the desired DNA into the cell.
The RNP-induced cutting at the target site activates the HSC repair machinery, leveraging the delivered DNA as a template to meticulously mend the cut site. This process seamlessly incorporates the desired DNA sequence into the target site, ensuring a durable genetic repair. By harnessing the inherent cellular repair system, our approach stimulates stem cells to independently rectify disease-causing genetic mutations, providing a sustainable solution.
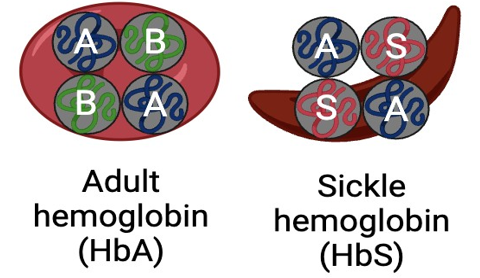
Our lead product candidate is a highly differentiated approach with the potential to directly correct the mutation that causes sickle cell disease (SCD) and restore normal adult hemoglobin (HgbA) expression.
Patients with sickle cell disease harbor a mutation within the beta-globin gene of HSCs. When RBCs mature from a mutation-carrying HSC, the RBC will harbor sickle hemoglobin S (HbS) rather than functional adult hemoglobin B (HbA). The HbS causes a disruption of normal protein-protein interactions. Hemoglobin S proteins form insoluble chains inside the cell, causing the RBC to morph into a “sickle” shape. Sickle cells are ineffective at binding and carrying oxygen and cannot circulate throughout the body. The result is that individuals with sickle cell disease experience many symptoms caused by dysfunctional, HbS-containing RBCs.
Temporary introduction of the RNP and viral delivery system provide specific, durable genetic repair. Kamau’s HDR technology harnesses the cell’s own repair system, prompting stem cells to repair disease-causing genetic mutations themselves. By directly correcting the sicklecausing mutation within HSCs, daughter RBCs also carry the corrected DNA sequence. This restores the normal balance of alpha-globin and beta-globin expression, resulting in normal, oxygen-carrying RBCs
These technological advantages go beyond first generation CRISPR-Cas9 approaches
HDR technology enhances a cell’s own repair system, prompting stem cells to repair themselves.
This platform builds from first generation CRISPR-Cas9 technology, by not only cutting DNA, but also providing a template to repair DNA.
The unique ability to modify genetic material from 1bp up to 4000bp gives our technology the ability to precisely repair, replace, or insert genes.
Increase in the desired gene from 10% to 30-50% across a wide range of gene targets and a variety of DNA templates has been achieved.
High on-target editing was achieved while off-target editing remains less than 1%.
This process is highly engineered to interlock, significantly reducing manufacturing burden.
HDR technology enhances a cell’s own repair system, prompting stem cells to repair themselves.
Cas9
gRNA
AAV6
DNA template
Kamau is bringing groundbreaking research into the clinic to impact patient outcomes. See below publications underlying our work in gene therapy and diving into the components of our technology and the strategic decisions impacting our work.
The β-haemoglobinopathies, such as sickle cell disease and β-thalassaemia, are caused by mutations in the β-globin (HBB) gene and affect millions of people worldwide.
As CRISPR-based therapies enter the clinic, evaluation of safety remains a critical and active area of study. Here, we employ a clinical next generation sequencing (NGS)…
Permanent modification of the human genome in vivo is impractical owing to the low frequency of homologous recombination in human cells, a fact that hampers..
CRISPR-Cas-mediated genome editing relies on guide RNAs that direct site-specific DNA cleavage facilitated by the Cas endonuclease. Here we report that chemical..
Bacteria and archaea protect themselves from invasive foreign nucleic acids through an RNA-mediated adaptive immune system called CRISPR…
CRISPR-Cas9 technology enables precision engineering of the genome with single base-pair resolution.1 The site specificity of this system…
Translation of the CRISPR–Cas9 system to human therapeutics holds high promise. However, specificity remains a concern especially when modifying stem cell populations…
Therapeutic applications of nuclease-based genome editing would benefit from improved methods for transgene integration via homology-directed repair (HDR)..
Graft failure occurs in approximately 20% of patients after unrelated umbilical cord blood transplantation (UCBT). This could be because of inadequate potency of the cord blood..
CRISPR/Cas9 repair of CYBB mutations with an oligodeoxynucleotide restores endogenous regulation of expression in X-CGD CD34+ cells…
3519-Pos Board B247 Universal Behavior of DNA Escape, Drift, and Diffusion in Nanopores David P. Hoogerheide1,2, Jene A. Golovchenko3. 1 NIST Center for Neutron Research…
To investigate the impact of post-thaw CFU-GM counts on the quality of umbilical cord blood (UCB), we studied transplant outcomes in 269 patients receiving single…
Kamau Therapeutics technology includes intellectual property and proprietary technology initially developed at Stanford University and Graphite Bio through to a clinical-stage program, and now refocused at Kamau Therapeutics with the conviction that targeted gene integration will lead to an entirely new class of potentially curative therapies. Predicated on additional manufacturing advances and clinical learnings, the Kamau Therapeutics team has the depth of expertise with this gene correction platform to successfully advance the improved technology through further clinical evaluation towards FDA-approval.